How connected are deep and shallow Pearl Oysters in the Eighty Mile Beach area?
Background - Why Eighty Mile Beach - What we did - What we found - References - Acknowledgements
Background
Sustainable fisheries management relies heavily on an accurate understanding of stock-recruitment dynamics and patterns of connectivity in order to set biologically relevant spatial boundaries and harvest limits. Many marine invertebrate species, including the commercially important silverlip pearl oyster, have a bipartite life cycle which consists of a relatively sessile adult stage and a dispersing larval stage. How far and where larvae will dispersal is governed by the complex interplay between larval biology (e.g. how well larvae can swim, how long they live for) and oceanographic processes including tidal currents and flows. While understanding where the larvae go is critical for managing fisheries and populations, directly tracking millions of tiny larvae as they disperse in the ocean is near impossible! Instead, we use genetics and look at changes in allele frequencies between populations from different sample sites to infer dispersal patterns.
Why Eighty Mile Beach?
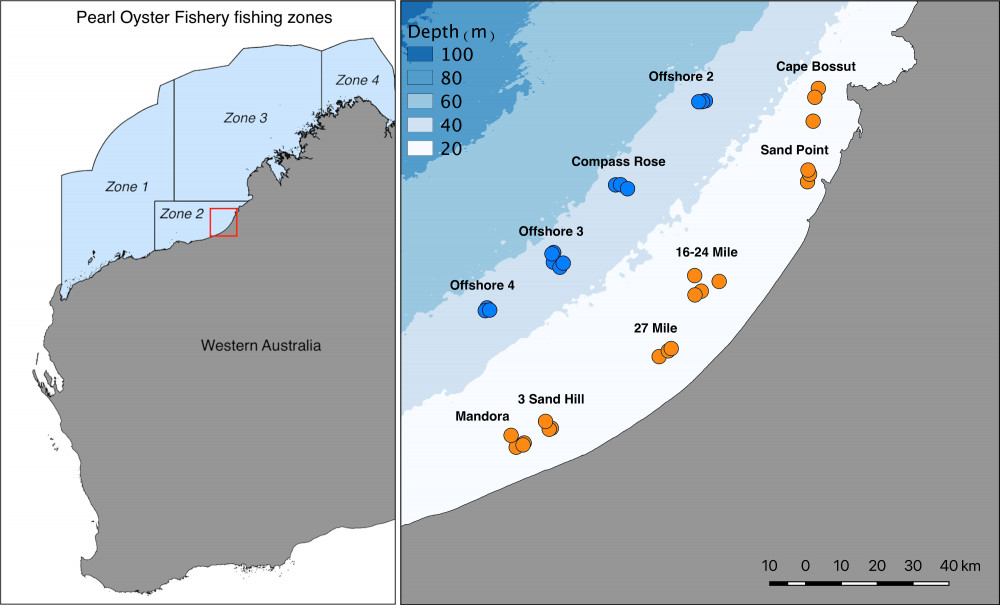
Pinctada maxima is the largest of the Pinctada species used for pearl production1, and the primary species used for culturing pearls in Australia. Pearl production in Australia relies heavily on wild-caught individuals, and the Australian fishery represents the last remaining wild-capture pearl oyster fishery in the world. Each year, approximately 500,000 individuals are harvested from the wild, with the majority of fishing focused in Zone 2 along Eighty-Mile Beach in Western Australia (Figure 1). Wild oysters are collected by divers on surface-supplied air and transported to commercial aquaculture farms for pearl production. Commercial diving for pearl oysters occurs predominantly in nearshore habitats in 8-15 metres depth, and it is thought that the deep offshore populations (>35m depth) may act as broodstock, providing recruits into the shallow fished areas along Eighty Mile Beach.
What we did
Tissue samples from a total of 715 pearl oysters were collected from 11 offshore and 22 inshore sites in the Eighty Mile Beach area by commercial divers (Figure 1). All samples collected from inshore (n=467) were from regular fishing grounds. Samples collected offshore (n=248) included one deep fishing ground (Compass Rose) and three other areas where moderate densities of pearl oysters had been recorded during oyster habitat towed video and multibeam sonar surveys (https://northwestatlas.org/nwa/nws2s-oysters). We then extracted the DNA from these samples and used cutting-edge genetic sequencing techniques to infer levels of connectivity between deep and shallow Pearl Oysters (Figure 2).
What we found
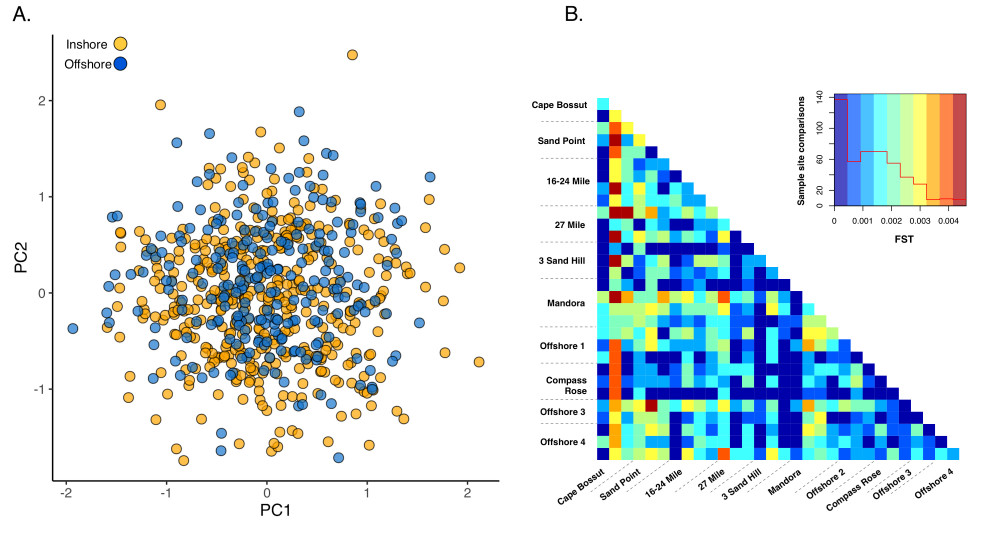
Our genetic data indicates that Pinctada maxima in the Eighty Mile Beach area constitutes a single genetic stock with high rates of dispersal between inshore and offshore sites (Figure 3). The patterns were consistent with the long larval duration of the species1, and the large tidal currents of the region2. The continental shelf in northwest Australia extends 100s of kilometres, resulting in some of the largest tides in the world3. These tides drive strong cross-shelf currents that could potentially carry P. maxima larvae tens of kilometres2 although particle dispersal modelling and spat surveys suggest that inshore populations along Eighty-Mile Beach are largely self-seeding, with spawning and recruitment concentrated along the 8-15 metre contour, and with intermittent dispersal offshore2. We were not able to identify any clear directionality to gene flow either onshore or offshore; however, our data clearly show that there is sufficient cross-shelf dispersal to homogenize the genetic structure of P. maxima in the region. It is likely that in a dynamic environment like Eighty Mile Beach, population structure is fluid and can change from year to year, and although significant differentiation arises in the population, successive dispersal and recruitment events over generations homogenize the population. As a result, the genetic patches are not discrete populations, but rather a small part of a larger genetic mosaic that fluctuates through time. Our results will inform sustainable management of the pearl oyster fishery by providing knowledge about the interconnectedness of populations that will enable managers to ensure sufficient larval production and recruitment to replenish fished populations.
References
1 Rose, R.A., and Baker, S.B. (1994). Larval and spat culture of the Western Australian silver- or goldlip pearl oyster, Pinctada maxima Jameson (Mollusca: Pteriidae). Aquaculture 126, 35–50.
2 Condie, S., and Hart, A. (2006). Transport and recuritment of silver-lip pearl oyster larvae on Australia’s North West Shelf. J. Shellfish Res. 25, 151–157.
3 Lowe, R.J., Leon, A.S., Symonds, G., Falter, J.L., and Gruber, and R. (2015). The intertidal hydraulics of tide-dominated reef platforms. J. Geophys. Res. Ocean. 120, 4845–4868.
Acknowledgements
This work was conducted as part of the North West Shoals to Shore Program, which is proudly supported by Santos as part of the company’s commitment to better understand WA’s marine environment. AIMS acknowledges Yawuru, Karajarri and Nyangumarta People as Traditional Owners of the 80 Mile Beach coast and surrounding region where this work on Pearl Oyster was undertaken for the North West Shoals to Shore Research Program. We recognise these People’s ongoing spiritual and physical connection to Country and pay our respects to their Aboriginal Elders past, present and emerging.’ We would like to thank Peter Farrell, Sabrina Arklie and Mark Chinkin at AIMS for support with project logistics and sample collection. We would also like to thank Paspaley Pearling Company for help with sample collection in inshore fishery areas and Paspaley for providing vessels and divers for offshore collections.



